Ingredient Descriptions + Safety + Why We Chose Each
1. Purified water.
INCI Name: Aqua/Water
2. Citric Acid. Our bodies are used to an acidic environment, and our scalp is no exception. We can minimize irritation by using citric acid to adjust the pH downwards. Also, the preservatives need an acidic environment to be active.
“Besides its role in pH adjustment, citric acid acts as a chelating agent. It binds with metal ions such as aluminum, calcium, and magnesium that may be present in water or other ingredients, preventing these ions from interfering with the performance of the product. By sequestering these metals, citric acid helps to improve the stability and efficacy of the product, ensuring consistent performance over time”.
INCI Name: Citric acid
Safety information: https://www.cir-safety.org/sites/default/files/citric032012FR.pdf
“Citric acid is an alpha hydroxy acid (AHA) commonly used in cosmetics as a pH adjuster and exfoliant. It has a low to moderate potential for skin irritation and sensitization, depending on the concentration and formulation. At low concentrations (up to 10%), citric acid is generally well-tolerated. However, higher concentrations can cause irritation, particularly on sensitive skin or with prolonged exposure. Repeated insult patch tests (RIPT) and clinical evaluations have shown that citric acid can be irritating at higher levels but is usually non-sensitizing at cosmetic use concentrations.
Citric acid exhibits low toxicity. Toxicological studies, including acute and sub-chronic toxicity evaluations in animals, have indicated that it is non-toxic when applied dermally or ingested. It is generally recognized as safe (GRAS) by the FDA for use in food, further supporting its safety profile in cosmetics.
There is no evidence to suggest that citric acid causes organ damage when used in cosmetic formulations. Systemic absorption through the skin is minimal, and studies have not indicated any adverse effects on internal organs with dermal application. The metabolism of citric acid in the body further reduces the risk of systemic toxicity.
Citric acid has not been found to be carcinogenic. Long-term studies and reviews by regulatory agencies, including the Cosmetic Ingredient Review (CIR) Expert Panel, have not identified any carcinogenic effects associated with its use in cosmetics. It is considered safe for topical application from a carcinogenicity perspective.
Citric acid is safe for use in cosmetic products at typical concentrations. It has a low to moderate potential for skin irritation, depending on the concentration, but is generally non-sensitizing. It is non-toxic at cosmetic use levels, poses no risk of organ damage, and is not carcinogenic. Proper formulation and concentration limits are essential to minimize the risk of irritation”.
3. Panthenol. “Panthenol, also known as provitamin B5, is a highly effective ingredient in hair and scalp serums due to its numerous benefits for hair and scalp health. When applied topically, panthenol converts into pantothenic acid, which access the hair shaft and scalp, providing long-lasting moisture. This moisture retention helps to strengthen the hair, reducing breakage and split ends, and promotes a healthier scalp environment. Panthenol also exhibits Panthenol also exhibits calming properties soothing the scalp and combating temporary discomfort. Additionally, it enhances the elasticity and sheen of the hair, giving it a fuller, more vibrant appearance. By incorporating panthenol into a hair serum, you can achieve improved hair resilience, thicker hair, and a healthier scalp, ultimately supporting optimal hair appearance”.
INCI Name: Panthenol
Safety information: https://www.cir-safety.org/sites/default/files/panthenol_0.pdf
“Panthenol, a provitamin of B5, is widely used in cosmetic formulations for its moisturizing properties. It is well-tolerated by the skin and has a low potential for causing irritation or sensitization. Multiple studies, including patch tests on human subjects, have demonstrated that panthenol is generally non-irritating and non-sensitizing at concentrations typically used in cosmetics (up to 5%).
Toxicological data indicate that panthenol is non-toxic when used in cosmetics. Acute toxicity studies in animals have shown that panthenol has a high LD50, indicating low toxicity. Dermal application in animal models has not resulted in significant toxic effects, confirming its safety profile for topical use.
There is no evidence to suggest that panthenol causes organ damage when used in cosmetic formulations. Long-term dermal application studies have not demonstrated any adverse effects on organs. Systemic absorption of panthenol through the skin is minimal, further reducing the risk of organ toxicity.
Panthenol is not carcinogenic. Comprehensive evaluations, including long-term studies and reviews by regulatory bodies, have not identified any carcinogenic effects associated with panthenol. It is considered safe for use in cosmetics from a carcinogenicity standpoint.
Panthenol is a safe ingredient for use in cosmetic products. It has a low potential for skin irritation and sensitization, is non-toxic, poses no risk of organ damage, and is not carcinogenic. Its extensive use in a wide range of cosmetic products supports its safety profile”.
4. Green tea extract. “Camellia Sinensis, commonly known as green tea leaf extract, offers a multitude of benefits for hair and scalp health. Rich in polyphenols, particularly catechins, green tea extract possesses powerful antioxidant and calming properties that help to protect the scalp and hair follicles from damage. These antioxidants help protect hair from environmental stressors, reducing stress that can weaken hair and impede hair health. Green tea extract also promotes better nutrition for optimal hair health, ensuring that hair follicles receive adequate nutrients. Additionally, green tea is known to help balance natural oils in skin with excess oiliness. By incorporating Camellia Sinensis leaf extract into a hair serum, you can enhance the overall health of the scalp and hair, fostering a conducive environment for robust hair appearance.
Green tea extract's potent calming properties also contribute to soothing the scalp combating temporary discomfort. Moreover, the catechins in green tea have been known to nourish hair follicles encouraging them to produce stronger, thicker hair. The combination of these benefits makes green tea extract an excellent ingredient for a hair health serum, promoting a healthy scalp environment and supporting the overall health and vitality of the hair”.
INCI Name: Camellia sinensis (Green tea) leaf extract
Safety information: https://www.cir-safety.org/sites/default/files/Camell_032014_Rep.pdf
“Camellia sinensis leaf extract, derived from the leaves of the tea plant, is widely used in cosmetics for its antioxidant and soothing properties. Studies have shown that this extract has a low potential for skin irritation and sensitization. Repeated insult patch tests (RIPT) and other dermal safety assessments on human subjects have demonstrated that it is generally non-irritating and non-sensitizing at typical cosmetic use concentrations (up to 5%).
Camellia sinensis leaf extract exhibits low toxicity. Toxicological studies, including acute and chronic toxicity evaluations in animal models, have indicated that it is non-toxic when applied topically. The extract is rich in polyphenols, particularly catechins, which are not associated with significant adverse effects at cosmetic concentration.”
There is no evidence to suggest that Camellia sinensis leaf extract causes organ damage when used in cosmetic formulations. Systemic absorption through the skin is minimal, and no adverse effects on internal organs have been reported in dermal application studies. The safety of long-term use has been supported by the absence of organ toxicity in relevant studies.
Camellia sinensis leaf extract has not been found to be carcinogenic. Comprehensive reviews and long-term studies have not identified any carcinogenic effects associated with its use in cosmetics. The antioxidant properties of the extract, primarily due to its polyphenol content, may provide a protective effect.
Camellia sinensis leaf extract is considered safe for use in cosmetic products. It has a low potential for skin irritation and sensitization, is non-toxic, poses no risk of organ damage, and is not carcinogenic. Its widespread use in a variety of cosmetic formulations underscores its favorable safety profile”.
5. Xanthan gum
Safety information: https://www.cir-safety.org/sites/default/files/microb092012rep.pdf
“Xanthan gum, a polysaccharide used as a thickening and stabilizing agent in cosmetics, is generally recognized as having low skin irritation potential. Clinical studies and patch tests on human volunteers show that xanthan gum is non-irritating at typical use concentrations, which range from 0.1% to 2%. Its gentle nature makes it suitable for products formulated for sensitive skin.
Xanthan gum is considered to have a low potential for sensitization. Human repeated insult patch tests (HRIPT) and maximization tests have demonstrated that xanthan gum does not induce sensitization reactions. Additionally, animal studies support these findings, showing no significant sensitizing effects.
Xanthan gum exhibits low acute toxicity. Oral toxicity studies in rodents report an LD50 greater than 45,000 mg/kg, indicating minimal risk from accidental ingestion. It is also used as a food additive, further supporting its safety for human consumption. Dermal toxicity studies show no adverse effects even at high concentrations, highlighting its safety for topical application.
Subchronic exposure studies in animals have shown that xanthan gum is well-tolerated with no significant adverse effects on organs or overall health. In a 90-day dietary study in rats, no toxicologically significant effects were observed at doses up to 1,000 mg/kg/day. Chronic toxicity data are limited, but available studies suggest a low risk of chronic toxicity.
Xanthan gum is not absorbed through the skin in significant amounts, reducing the risk of systemic exposure and organ damage. It is primarily excreted unchanged in the feces when ingested. Animal studies, including those involving repeated dosing, have not demonstrated significant organ toxicity. Clinical observations in human subjects using xanthan gum-containing products have not indicated any adverse effects on internal organs.
There is no evidence to suggest that xanthan gum is carcinogenic. It is not classified as a carcinogen by the International Agency for Research on Cancer (IARC), the U.S. Environmental Protection Agency (EPA), or other major regulatory bodies. Long-term animal studies have not demonstrated an increased incidence of tumors, supporting the absence of carcinogenic risk.
In vitro and in vivo genotoxicity tests, including bacterial reverse mutation assays (Ames test) and mammalian chromosomal aberration tests, have demonstrated that xanthan gum is non-genotoxic. These studies indicate that xanthan gum does not pose a risk of genetic damage.
Xanthan gum is considered safe for use in cosmetic formulations based on its low potential for skin irritation and sensitization, minimal toxicity, lack of organ damage, and absence of carcinogenic and genotoxic effects. Its favorable safety profile supports its widespread use in various personal care products, including those intended for sensitive skin”.
6. Ethoxydiglycol. “Ethoxydiglycol, also known as diethylene glycol monoethyl ether, is a versatile enhancer commonly used in hair health serums. Its primary function is to improve the solubility of various active ingredients, ensuring they remain evenly distributed and stable within the formulation. This helps to enhance the overall efficacy of the serum by ensuring that each application delivers a consistent and potent dose of active ingredients to the scalp.
As a known enhancer, ethoxydiglycol plays a crucial role in facilitating the absorption of active cosmetic ingredients into the scalp and hair follicles. This increased bioavailability means that ingredients like peptides, vitamins, and plant extracts into the scalp, where they can exert their beneficial effects more effectively. By improving the delivery of these active compounds, ethoxydiglycol helps to maximize hair and scalp health.
Additionally, ethoxydiglycol has a low viscosity, which contributes to a lighter, non-greasy texture in the serum. This makes the product easier to apply and distribute evenly across the scalp, enhancing the user experience. Its compatibility with a wide range of cosmetic ingredients also ensures that the serum remains stable and effective throughout its shelf life.
Incorporating ethoxydiglycol into a hair a health serum enhances the product's overall performance, ensuring that the active ingredients are efficiently delivered to the scalp, ultimately supporting healthier and stronger hair”.
INCI Name: Ethoxydiglycol
Safety information: https://www.cir-safety.org/sites/default/files/ADIOLS042017TR.pdf
Potassium sorbate. “Potassium sorbate is a mild and effective preservative that complements other preservatives like phenoxyethanol and sodium benzoate in hair serums. It helps to inhibit the growth of mold, yeast, and fungi, ensuring the serum remains free from microbial contamination. This preservative is known for its low toxicity and high efficacy, making it a safe choice for use in cosmetic products, especially those applied to sensitive areas like the scalp. Potassium sorbate's inclusion in a hair health serum helps to extend the product's shelf life while maintaining the stability and potency of the active ingredients. This ensures that the serum can effectively promote a healthy scalp environment and support hair health over an extended period”.
INCI Name: Potassium sorbate
Safety information: https://journals.sagepub.com/doi/10.3109/10915818809078711
“Potassium sorbate is a preservative commonly used in cosmetics to inhibit the growth of mold, yeast, and bacteria. It has a low potential for skin irritation and sensitization. Repeated insult patch tests (RIPT) and other dermal safety evaluations on human subjects have demonstrated that potassium sorbate is generally non-irritating and non-sensitizing at typical cosmetic use concentrations (up to 0.5%).
Potassium sorbate exhibits low toxicity. Acute toxicity studies in animals have shown that it has a high LD50, indicating low toxicity when ingested or applied dermally. Chronic toxicity studies have also supported its safe use in cosmetics, with no significant adverse effects reported at typical use levels.
There is no evidence to suggest that potassium sorbate causes organ damage when used in cosmetic formulations. Systemic absorption through the skin is minimal, and studies have not shown any adverse effects on internal organs. Its use at low concentrations in cosmetics further mitigates any potential risk of systemic toxicity.
Potassium sorbate has not been found to be carcinogenic. Long-term studies and reviews by regulatory agencies, including the Cosmetic Ingredient Review (CIR) Expert Panel, have not identified any carcinogenic effects associated with its use in cosmetics. It is considered safe for topical application from a carcinogenicity perspective.
Potassium sorbate is safe for use in cosmetic products. It has a low potential for skin irritation and sensitization, is non-toxic at cosmetic use levels, poses no risk of organ damage, and is not carcinogenic. Its extensive use as a preservative in a wide range of cosmetic formulations supports its favorable safety profile”.
Anagain. “Pisum Sativum (Pea) Sprout Extract is a powerful ingredient known for its ability to support optimum hair health. Rich in phytonutrients, this extract helps to support the growth phase of the hair cycle. The active compounds in pea sprout extract have been shown to nourish hair follicles, strengthen the hair shaft, and support the overall resilience of the hair. this extract ensures that hair follicles receive essential nutrients, promoting healthier and stronger hair. Incorporating Pisum Sativum sprout extract in a hair health serum can result in visibly thicker and more robust hair, making it an excellent choice for those seeking to support hair appearance”.
INCI Name: Pisum sativum (Pea) sprout extract, alcohol, aqua
Safety information: Not yet evaluated by the CIR
“Pisum Sativum sprout extract, combined with alcohol and water, is used in cosmetics for its purported hair health and skin conditioning benefits. The extract itself has a low potential for skin irritation and sensitization. However, the presence of alcohol can increase the potential for irritation, especially on sensitive skin or in high concentrations. Patch tests on human subjects have shown that Pisum Sativum sprout extract is generally non-irritating and non-sensitizing at typical use concentrations (up to 2%), though formulations should be carefully balanced to minimize the irritation potential of alcohol.
Pisum Sativum sprout extract is non-toxic when used in cosmetic formulations. Toxicological studies have shown that pea sprout extract does not exhibit significant toxicity. The concentrations used in cosmetics are well within safe limits, and alcohol, while potentially irritating, is not toxic at the low concentrations typically used in topical formulations.
There is no evidence to suggest that Pisum Sativum sprout extract causes organ damage when used topically. Systemic absorption through the skin is minimal, and no adverse effects on internal organs have been reported in dermal application studies. Alcohol, although systemically absorbed more readily, does not cause organ damage at the concentrations used in these formulations.
Pisum Sativum sprout extract has not been found to be carcinogenic. Reviews and long-term studies have not identified any carcinogenic effects associated with its use in cosmetics. Similarly, the concentrations of alcohol used in cosmetic products are not associated with carcinogenicity.
Pisum Sativum (pea) sprout extract, when combined with alcohol and water, is considered safe for use in cosmetic products at typical concentrations. It has a low potential for skin irritation and sensitization when formulated correctly, is non-toxic, poses no risk of organ damage, and is not carcinogenic. Formulations should ensure that alcohol levels are kept within safe limits to minimize irritation potential”.
7. Hexapeptide-11. “Hexapeptide-11 is a bioactive peptide that offers significant benefits for hair health by nourishing and providing key elements in the scalp. It helps to support hair appearance and strength. Hexapeptide-11 supports elasticity and texture of the hair, making it more resilient to damage and breakage. By supporting the natural hair growth cycle and enhancing the overall health of the scalp, this peptide helps to create a conducive environment for robust hair health. Including hexapeptide-11 in a hair health serum can result in stronger, thicker, and more vibrant hair, addressing common issues related to hair thinning”.
INCI Name: Water/Aqua, hexapeptide-11 Safety information: Not yet evaluated by the CIR
“Hexapeptide-11 is a synthetic peptide used in cosmetics for its anti-aging and skin-conditioning properties. It is generally well-tolerated by the skin and has a low potential for irritation and sensitization. Clinical evaluations and patch tests have shown that hexapeptide-11, when used in cosmetic formulations at concentrations up to 5%, does not cause significant irritation or sensitization.
Hexapeptide-11 exhibits low toxicity. Toxicological assessments, including acute and chronic toxicity studies, have indicated that it is non-toxic at the concentrations typically used in cosmetics. The peptide's large molecular size reduces its ability to access the skin, which contributes to its low systemic toxicity.
There is no evidence to suggest that hexapeptide-11 causes organ damage when used in cosmetic formulations. Systemic absorption through the skin is minimal, and studies have not reported adverse effects on internal organs following dermal application. The use of hexapeptide-11 in cosmetics at low concentrations mitigates any potential risk of systemic toxicity.
Hexapeptide-11 has not been found to be carcinogenic. Long-term studies and reviews by regulatory bodies have not identified any carcinogenic effects associated with its use in cosmetics. It is considered safe for topical application from a carcinogenicity standpoint.
Hexapeptide-11 solution is safe for use in cosmetic products at typical concentrations. It has a low potential for skin irritation and sensitization, is non-toxic, poses no risk of organ damage, and is not carcinogenic. Its favorable safety profile supports its use in a variety of anti-aging and skin-conditioning formulations”.
8. Propanediol. “Propanediol, also known as 1,3-propanediol, is a multifunctional ingredient that offers numerous benefits in scalp serums. It acts as a solvent, improving the solubility of active ingredients and enhancing their efficacy. Additionally, propanediol functions as a humectant, attracting and retaining moisture in the scalp, which helps to maintain hydration and prevent dryness. This can be particularly beneficial for individuals with dry or sensitive scalps. Propanediol can also act as a preservative. Its mildness makes it suitable for sensitive skin, reducing the risk of irritation. Furthermore, propanediol enhances the texture and feel of the serum, providing a smooth, non-greasy application. As a sustainable and eco-friendly ingredient derived from renewable sources, it aligns with the growing demand for environmentally conscious cosmetic products. Overall, propanediol improves the performance, stability, and user experience of scalp serums, making it a valuable addition to such formulations”.
INCI Name: Propanediol
Safety information: https://www.cir-safety.org/sites/default/files/aldiol032018FR.pdf
“Propanediol, also known as 1,3-propanediol, is widely recognized for its low skin irritation potential. Clinical studies and patch tests on human subjects indicate that propanediol is generally non-irritating at concentrations typically used in cosmetic formulations, which range from 1% to 10%. It is less irritating than propylene glycol, making it a preferred alternative in sensitive skin products.
Propanediol has a low potential for sensitization. In studies involving repeated application to human skin, propanediol did not induce sensitization reactions. Animal studies corroborate these findings, showing no significant sensitizing effects even at higher concentrations.
Propanediol exhibits low acute toxicity. Oral toxicity studies in rodents report an LD50 greater than 15,000 mg/kg, indicating minimal risk from accidental ingestion. Dermal toxicity studies also show low toxicity, with no adverse effects observed at concentrations up to 20%.
Subchronic exposure studies in rats and dogs have shown that propanediol is well-tolerated with no significant adverse effects on organs or overall health. Doses up to 5,000 mg/kg/day in rats over 90 days showed no toxicologically significant effects. Chronic exposure data are limited, but existing studies suggest a low risk of chronic toxicity.
Propanediol is rapidly absorbed and metabolized in the body, primarily converted to pyruvate, which enters the tricarboxylic acid (TCA) cycle and is further metabolized to carbon dioxide and water. This metabolic pathway suggests a low likelihood of organ accumulation or damage. No significant organ toxicity has been reported in animal studies, even at high doses.
Propanediol has not shown carcinogenic potential in available studies. It is not classified as a carcinogen by the International Agency for Research on Cancer (IARC), the U.S. Environmental Protection Agency (EPA), or other major regulatory bodies. Long-term studies in animals have not demonstrated an increased incidence of tumors, supporting its safety profile regarding carcinogenicity.
In vitro and in vivo genotoxicity tests, including bacterial reverse mutation assays (Ames test) and mammalian chromosomal aberration tests, have demonstrated that propanediol is non-genotoxic. These studies indicate that propanediol does not pose a risk of genetic damage.
Propanediol is considered safe for use in cosmetic formulations based on its low potential for skin irritation and sensitization, minimal toxicity, lack of organ damage, and absence of carcinogenic and genotoxic effects. Its favorable safety profile makes it a suitable ingredient for various personal care products, including those intended for sensitive skin”.
Euxyl PE9010 also known as Phenoxyethanol or Ethylhexylglycerin “EUXYL PE9010 is a widely used preservative blend in cosmetic formulations, including scalp serums, due to its broad-spectrum antimicrobial efficacy. It helps to prevent the growth of bacteria, yeast, and mold, which ensures the product remains safe and effective over time. This is particularly important for scalp serums, which are often exposed to conditions that can promote microbial growth, such as warm and humid environments. Additionally, EUXYL PE9010 is compatible with a wide range of other cosmetic ingredients, making it a versatile choice for formulators. Its low usage levels are sufficient to achieve preservation, minimizing the risk of irritation or sensitization. Furthermore, it is effective across a broad pH range, enhancing its utility in various formulations. Overall, EUXYL PE9010 provides reliable preservation, ensuring product stability and safety, which is crucial for maintaining scalp health and promoting the efficacy of the serum's active ingredients”.
INCI Name: Phenoxyethanol, ethylhexylglycerin
Safety information:
https://cir-reports.cir-safety.org/view-attachment/?id=c6da0d14-8d74-ec11-8943-002248 2f06a6
“Phenoxyethanol is generally considered to have low skin irritation potential. Studies indicate that at typical use concentrations (up to 1%), it does not cause significant skin irritation or sensitization. However, at higher concentrations, it may cause mild to moderate irritation.
Phenoxyethanol is metabolized in the body to phenoxyacetic acid and excreted via urine. Acute oral toxicity studies in rats show an LD50 of 1,400 to 2,800 mg/kg, indicating low acute toxicity. Subchronic exposure studies have shown no significant adverse effects on organs at doses up to 1,000 mg/kg/day.
Long-term exposure studies in animals have not demonstrated significant organ damage. Phenoxyethanol does not accumulate in the body, reducing the risk of chronic toxicity.
There is no evidence from animal studies to suggest that phenoxyethanol is carcinogenic. It is not listed as a carcinogen by the International Agency for Research on Cancer (IARC), the U.S. Environmental Protection Agency (EPA), or other regulatory bodies.
Ethylhexylglycerin is known for its low irritation and sensitization potential. It is used in cosmetics as a skin-conditioning agent and preservative enhancer. Dermal application studies in human subjects have shown that it is well-tolerated at typical use concentrations (up to 1%).
The acute oral toxicity of ethylhexylglycerin is low, with an LD50 of over 2,000 mg/kg in rats. Subchronic studies indicate that ethylhexylglycerin is metabolized and excreted without causing significant toxicity to internal organs at doses up to 500 mg/kg/day.
Long-term studies have shown no significant effects on organ function or structure. Ethylhexylglycerin does not bioaccumulate, and its metabolic byproducts are rapidly cleared from the body.
There is no evidence to suggest that ethylhexylglycerin is carcinogenic. It is not classified as a carcinogen by major health or regulatory agencies.
The combination of phenoxyethanol and ethylhexylglycerin in cosmetic formulations is considered to enhance the antimicrobial efficacy while maintaining a low potential for irritation and sensitization. Safety assessments indicate that the blend is well-tolerated by the skin and does not pose significant risks of toxicity, organ damage, or carcinogenicity at the concentrations typically used in cosmetics. The complementary properties of phenoxyethanol and ethylhexylglycerin provide a synergistic effect, ensuring product safety and stability”.
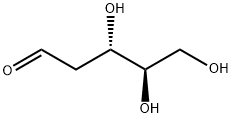
9. 2Ddr. This is the key sugar active.
INCI Name: 2-deoxy-D-ribose
10. Capilia Longa. This is a powerful plant ‘some based hair nutrient.
Informational link: https://www.vytrus.com/wp-content/uploads/P-CAPILIA-LONGA%E2%84%A2_EN_v22_01-4.pdf
INCI Name: Curcuma longa (Turmeric) callus conditioned media, aqua
Safety information: Not yet evaluated by the CIR
11. Cinnamic acid. This showed promising hair nutrient potential. It’s already used in cosmetics for its skin-conditioning qualities.
INCI Name: Cinnamic acid
12. Polysorbate-20. This serves to help dissolve the cinnamic acid.
INCI Name: Polysorbate-20
Safety information: https://www.cir-safety.org/sites/default/files/polysorbates_0.pdf
“Polysorbate 20, a nonionic surfactant and emulsifier commonly used in cosmetics, exhibits low skin irritation potential. Patch tests on human volunteers have shown minimal irritation at typical use concentrations, which range from 0.5% to 10%. Studies indicate that at these concentrations, Polysorbate 20 is well-tolerated by the skin, including sensitive skin types.
Polysorbate 20 is considered to have a low sensitization potential. Human repeated insult patch tests (HRIPT) and maximization tests have demonstrated that Polysorbate 20 does not induce sensitization reactions. Additionally, animal studies support these findings, showing no significant sensitizing effects.
Polysorbate 20 exhibits low acute toxicity. Oral toxicity studies in rodents report an LD50 greater than 25,000 mg/kg, indicating minimal risk from accidental ingestion. Dermal toxicity studies also show low toxicity, with no adverse effects observed even at high concentrations.
Subchronic exposure studies in animals have shown that Polysorbate 20 is well-tolerated with no significant adverse effects on organs or overall health. Repeated dose studies in rats and dogs have reported no toxicologically significant effects at doses up to 2,000 mg/kg/day. Chronic toxicity data are limited, but available studies suggest a low risk of chronic toxicity.
Polysorbate 20 is metabolized and excreted by the body, reducing the risk of organ accumulation and damage. Animal studies, including those involving repeated dosing, have not demonstrated significant organ toxicity. Clinical observations in human subjects using Polysorbate 20-containing products have not indicated any adverse effects on internal organs.
There is no evidence to suggest that Polysorbate 20 is carcinogenic. It is not classified as a carcinogen by the International Agency for Research on Cancer (IARC), the U.S. Environmental Protection Agency (EPA), or other major regulatory bodies. Long-term animal studies have not demonstrated an increased incidence of tumors, supporting the absence of carcinogenic risk.
In vitro and in vivo genotoxicity tests, including bacterial reverse mutation assays (Ames test) and mammalian chromosomal aberration tests, have demonstrated that Polysorbate 20 is non-genotoxic. These studies indicate that Polysorbate 20 does not pose a risk of genetic damage.
Polysorbate 20 is considered safe for use in cosmetic formulations based on its low potential for skin irritation and sensitization, minimal toxicity, lack of organ damage, and absence of carcinogenic and genotoxic effects. Its favorable safety profile supports its widespread use in various personal care products, including those intended for sensitive skin”.
13. Copper tripeptide. “GHK-Cu, or Copper Tripeptide-1, offers a range of benefits when used in scalp serums. This peptide complex, consisting of three amino acids bonded to a copper ion, is known for its remarkable ability to support scalp health. GHK-Cu provides essential components for maintaining healthy skin and hair follicles. Additionally, GHK-Cu has calming properties. It can also promotes better nutrition for optimal hair health, ensuring that hair follicles receive essential nutrients. Furthermore, GHK-Cu exhibits antioxidant properties protecting hair from environmental stressors. By incorporating GHK-Cu into scalp serums, users can benefit from By incorporating GHK-Cu into scalp serums, users can benefit from overall support scalp health, leading to stronger, healthier hair.”
INCI Name: GHK-Cu (glycyl-l-histidyl-l-lysine)
Safety information: https://www.cir-safety.org/sites/default/files/tripep032014tent.pdf
“GHK-Cu (Copper Tripeptide-1), a naturally occurring copper complex of the tripeptide glycyl-L-histidyl-L-lysine, is recognized for its low irritation potential. Clinical studies and patch tests have shown that GHK-Cu is non-irritating to human skin at typical use concentrations, which range from 0.01% to 2%. Its application in various skincare products, including those for sensitive skin, is generally well-tolerated.
The sensitization potential of GHK-Cu is low. Human repeated insult patch tests (HRIPT) have demonstrated that GHK-Cu does not induce sensitization reactions. Additionally, animal studies corroborate these findings, showing no significant sensitizing effects.
GHK-Cu exhibits low acute toxicity. Oral toxicity studies in animals indicate an LD50 greater than 5,000 mg/kg, suggesting minimal risk from accidental ingestion. Dermal toxicity studies have also shown no adverse effects at concentrations commonly used in cosmetic formulations, supporting its safety for topical application.
Subchronic exposure studies have shown that GHK-Cu is well-tolerated in animals, with no significant adverse effects on organs or overall health. Long-term exposure data are limited, but existing studies and clinical observations suggest a low risk of chronic toxicity.
GHK-Cu is metabolized and excreted efficiently, minimizing the risk of organ accumulation and damage. Studies in animals and clinical observations in humans have not demonstrated significant organ toxicity. GHK-Cu's metabolic pathway involves hydrolysis of the peptide, followed by the incorporation of copper into normal physiological processes.
There is no evidence to suggest that GHK-Cu is carcinogenic. It is not classified as a carcinogen by the International Agency for Research on Cancer (IARC), the U.S. Environmental Protection Agency (EPA), or other major regulatory bodies. Long-term studies have not indicated an increased incidence of tumors associated with GHK-Cu.
In vitro and in vivo genotoxicity tests, including bacterial reverse mutation assays (Ames test) and mammalian chromosomal aberration tests, have demonstrated that GHK-Cu is non-genotoxic. These studies indicate that GHK-Cu does not pose a risk of genetic damage.
GHK-Cu (Copper Tripeptide-1) is considered safe for use in cosmetic formulations based on its low potential for skin irritation and sensitization, minimal toxicity, lack of organ damage, and absence of carcinogenic and genotoxic effects. Its favorable safety profile supports its use in a variety of skincare products, including those designed for sensitive skin and anti-aging treatments”.